A First Look at Genetic and Biomarker Testing in the Disease Journey of RARE Registry Participants
A First Look at Genetic and Biomarker Testing in the Disease Journey of RARE Registry Participants
By: Precious Ngnosse & Jessica Scales
One major focus of cancer research is genetics–the study of changes in DNA that can drive the development of cancer. These genetic alterations may be inherited (helping to explain why cancer can run in families) or acquired over an individual’s lifetime. One way that physicians may diagnose and characterize cancer in their patients is by using tests that look at changes in DNA–such as gene mutations–that occur in healthy cells (inherited mutations) and cancer cells (acquired mutations). An understanding of these genetic alterations is important for informing cancer risk, diagnosis, prognosis, and treatment.
Compared to cutaneous (skin) melanoma, much less is known about the genetic risk factors (inherited mutations) or genetic tumor biomarkers (acquired mutations) present in patients with rare melanoma subtypes such as acral and mucosal melanoma. To help advance research in this area, the Melanoma Research Alliance (MRA) sponsored the first direct-to-patient registry called RARE to collect information on the patient perspective and disease journey from participants with cutaneous, acral, or mucosal melanoma. Using patient-reported data from the RARE Registry, we have examined for the first time the use of genetic and biomarker testing in the medical journeys of participants diagnosed with cutaneous, acral, or mucosal melanoma.
Genetic Testing: A Closer Look at Inherited Gene Mutations in Melanoma
Genetic testing is used to identify inherited changes in genes of healthy cells that may increase an individual’s risk for developing specific types of cancer. For a subset of melanomas, inherited changes in genes may increase an individual’s lifetime risk of developing the disease.
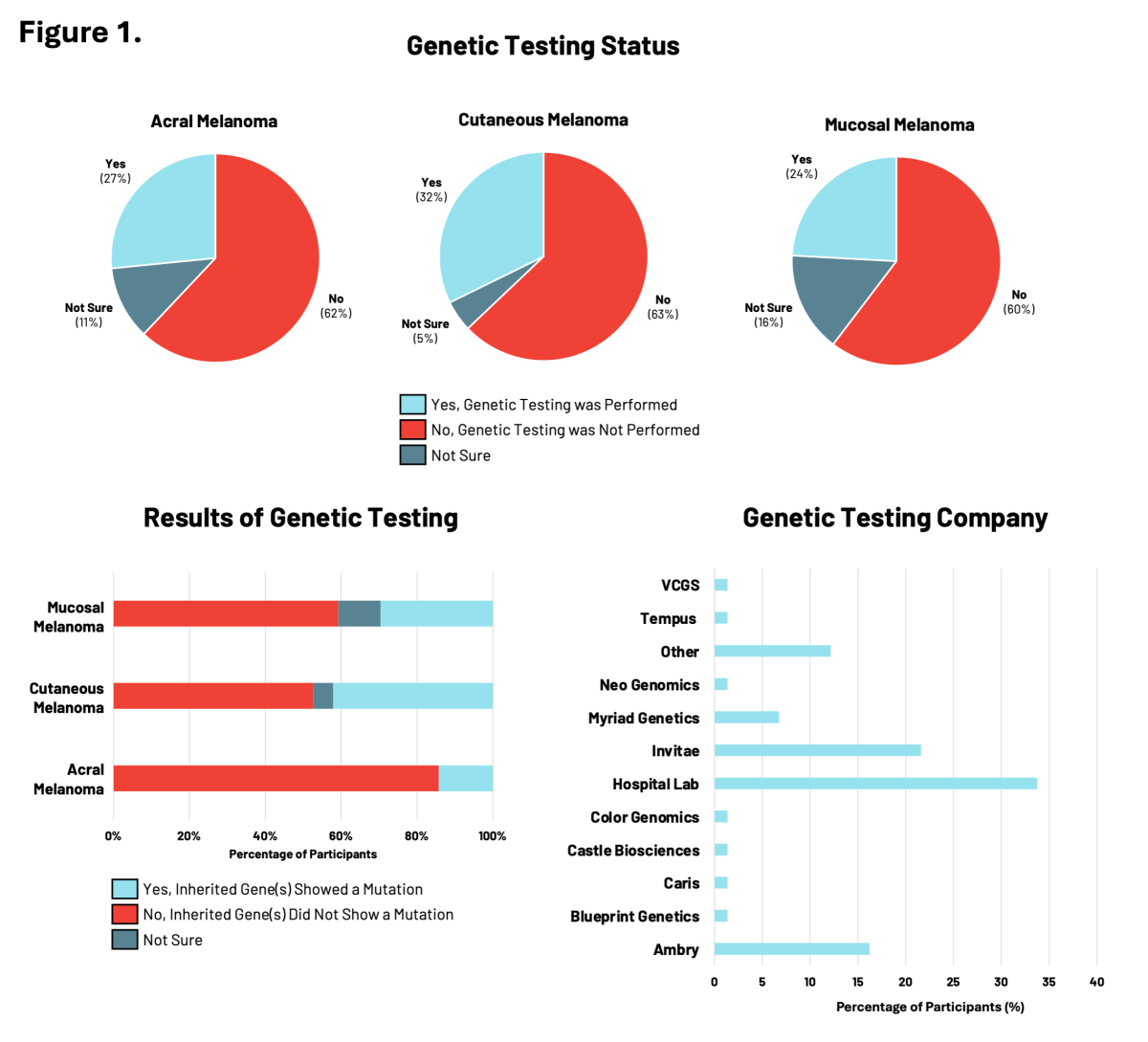
Given the importance of genetic testing in assessing melanoma risk and the RARE Registry’s goal of advancing knowledge about hereditary melanoma across all subtypes, we looked at the use of genetic testing by participants enrolled in RARE (Figure 1).
Among participants diagnosed with acral, cutaneous, or mucosal melanoma, between 24% to 32% reported having genetic testing. Of these participants, genetic testing results revealed the presence of inherited gene mutations, including nearly 42% of participants with cutaneous melanoma. Genetic testing showed that inherited mutations in cancer risk genes were overlapping yet distinct across the three melanoma subtypes. For all melanoma subtypes, participants accessed genetic testing most frequently through a hospital laboratory, followed by genetic testing companies like Ambry and Invitae.
Tumor Biomarker Testing: Decoding Your Melanoma
One type of tumor biomarker testing looks at the genetic changes in DNA or genes present in cancer cells (acquired mutations) and helps physicians to understand more about a patient’s specific cancer, including prognosis and personalized treatments. Studies of genetic tumor biomarkers in acral and mucosal melanomas are emerging: these rare subtypes have been found to harbor fewer acquired mutations and have distinct mutational profiles compared to cutaneous melanomas.
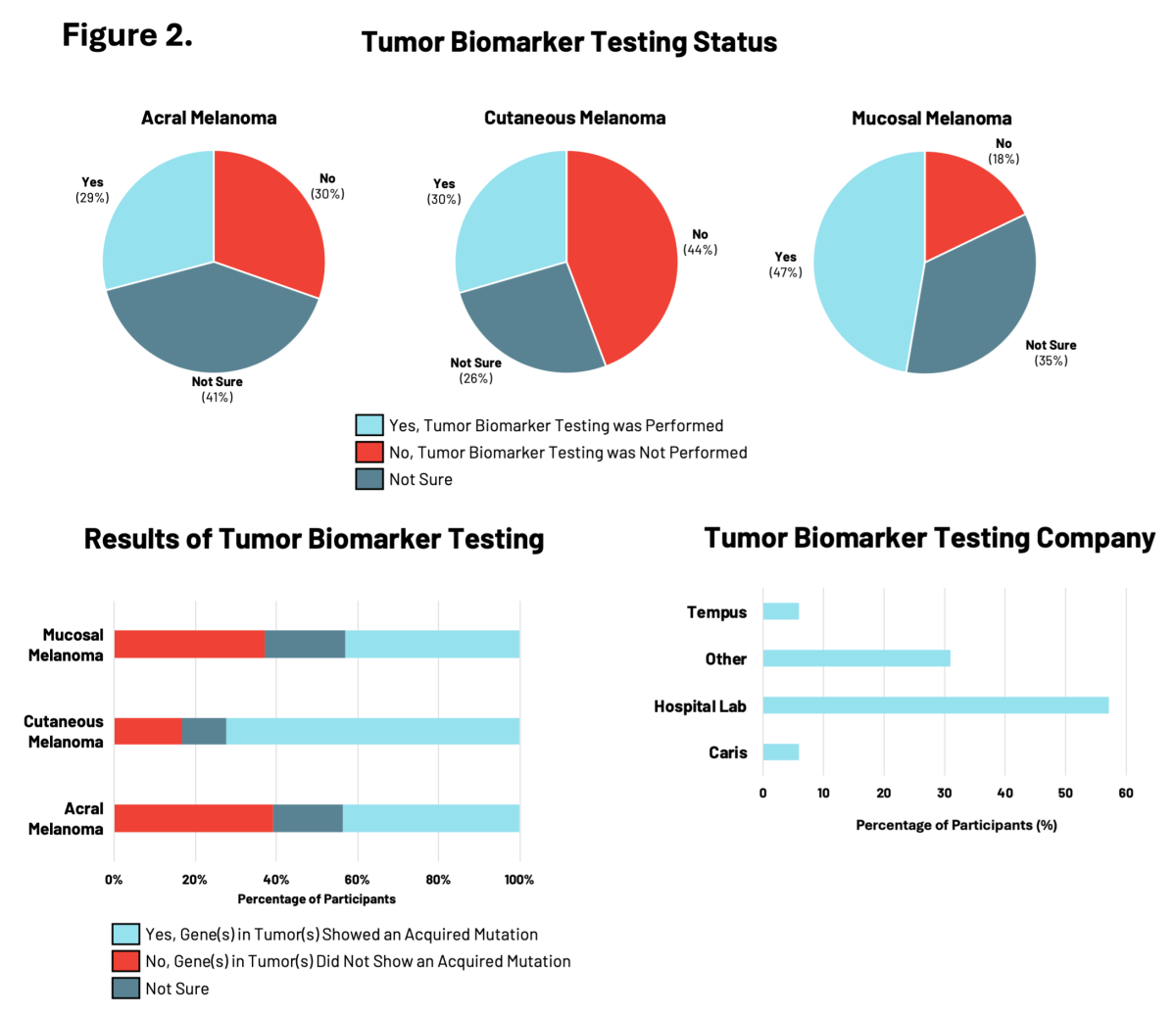
RARE Registry participants were asked whether their melanoma tumors had biomarker testing performed that specifically looked for acquired (not inherited) gene mutations in the DNA of melanoma tumor cells. For patients with melanoma, understanding the genetic changes acquired by the tumor cells is important for making treatment decisions.
In the RARE registry, participants with mucosal melanoma were the most likely to have had tumor biomarker testing performed (47%), compared to those with acral or cutaneous melanomas (<30%) (Figure 2). Of these participants, tumor biomarkers were identified in 72% of individuals with cutaneous melanoma, compared to approximately 43% of individuals with either acral or mucosal melanoma. Differences in the specific tumor biomarkers found by testing were observed across all three melanoma subtypes. Hospital-affiliated labs were most commonly used for tumor biomarker testing, followed by clinical diagnostic companies like Tempus, Caris, and others.
Blood Biomarker Testing: Monitoring Your Melanoma
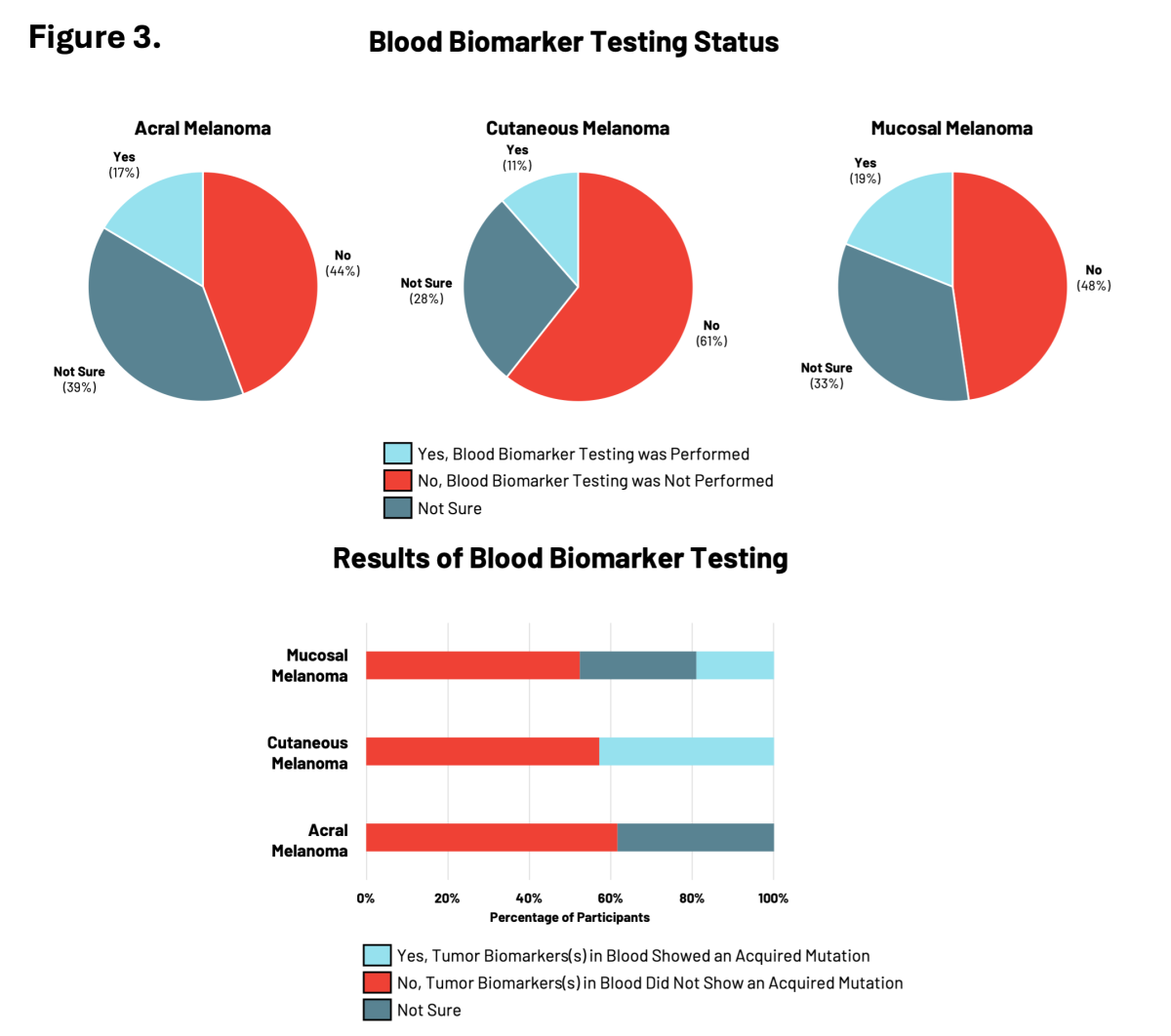
Blood biomarker testing measures specific molecules in blood samples (such as DNA) to monitor the progression of an individual’s cancer and responses to particular treatments. This is a newer type of testing that is starting to be used by physicians to monitor how well a treatment is working in patients with cancer.
For RARE registry participants, blood biomarker testing was the least common type of testing performed relative to genetic and tumor biomarker testing with between 11% to 19% of participants having had testing performed (Figure 3). Participants with cutaneous or mucosal melanoma reported the presence of tumor biomarkers showing acquired mutations in cancer risk genes.
Collectively, this initial look at RARE Registry data indicates that this patient-driven platform is valuable for capturing meaningful data across melanoma subtypes, and can enable collaborative efforts between patients and researchers to advance knowledge and use of genetic and biomarker testing in both common and rare melanoma subtypes.
Additional Resources and Ways to Get Involved
These findings represent the first in a series of insights emerging from MRA’s RARE Registry. Interested in participating in the RARE Registry? Learn more about how to become involved in this initiative by visiting raremelanoma.org.
Visit MRA’s dedicated resource pages to learn more about genetic and biomarker testing. If you are interested in genetic and biomarker testing, speak to your healthcare provider about potential options. It is important to ask what information these types of testing can provide, the limitations of such tests, and how testing can impact your current and future care.



 MRA Meet-ups are a series of live webinars designed specifically to educate and empower patients and caregivers. Several with specific application for RARE melanomas are showcased below and full repository of recorded MRA Meet Ups can be found
MRA Meet-ups are a series of live webinars designed specifically to educate and empower patients and caregivers. Several with specific application for RARE melanomas are showcased below and full repository of recorded MRA Meet Ups can be found  The Melanoma Research Alliance (MRA) convened in February for its 2025 Scientific Retreat and Melanoma Exchange Patient Forum in Washington, D.C., bringing together more than 300 participants from across the melanoma community. One of the focus areas of the Retreat was advancing research and care for rare melanoma subtypes. In addition to several scientific talks on rare melanomas, MRA hosted a cross-sector industry roundtable, “Harnessing Collective Power for Rare Melanomas,” involving 35 representatives across industry, academia, and patient advocacy groups. This roundtable explored key issues for rare melanoma patients, including drug access, clinical trials inclusion, health insurance denials of melanoma therapies, and model development for advancing rare melanoma research. The meeting produced actionable strategies that are detailed in the
The Melanoma Research Alliance (MRA) convened in February for its 2025 Scientific Retreat and Melanoma Exchange Patient Forum in Washington, D.C., bringing together more than 300 participants from across the melanoma community. One of the focus areas of the Retreat was advancing research and care for rare melanoma subtypes. In addition to several scientific talks on rare melanomas, MRA hosted a cross-sector industry roundtable, “Harnessing Collective Power for Rare Melanomas,” involving 35 representatives across industry, academia, and patient advocacy groups. This roundtable explored key issues for rare melanoma patients, including drug access, clinical trials inclusion, health insurance denials of melanoma therapies, and model development for advancing rare melanoma research. The meeting produced actionable strategies that are detailed in the  Cancer immunotherapy, particularly immune checkpoint inhibitors (ICIs), has transformed the treatment landscape for patients with advanced melanoma. While many have benefited, a significant portion of patients do not respond or eventually experience disease progression, highlighting the need for new options. One promising approach is tumor-infiltrating lymphocyte (TIL) therapy, a personalized, cell-based immunotherapy that harnesses a patient’s own immune cells to target and destroy melanoma tumors. Some patients treated with TIL therapy have experienced long-lasting or even curative responses.
Cancer immunotherapy, particularly immune checkpoint inhibitors (ICIs), has transformed the treatment landscape for patients with advanced melanoma. While many have benefited, a significant portion of patients do not respond or eventually experience disease progression, highlighting the need for new options. One promising approach is tumor-infiltrating lymphocyte (TIL) therapy, a personalized, cell-based immunotherapy that harnesses a patient’s own immune cells to target and destroy melanoma tumors. Some patients treated with TIL therapy have experienced long-lasting or even curative responses.  Despite the tremendous progress made in the last decade in treating melanoma, approximately one third of patients don’t benefit from currently approved therapies. This includes the majority of people with rare forms of melanoma — melanomas that develop in the eye, nailbeds, palms, soles of the feet, and various mucosal membranes. Patients facing these subtypes tend not to respond as well to current treatments as patients with cutaneous melanomas that arise on sun-exposed skin. But as investigators continue to chip away at understanding what causes these melanomas and how they differ from UV-driven cutaneous melanomas, the hope is the better understanding they reap will be sown into improved treatments for these patients. Researchers explored both the challenges and the opportunities for rare melanoma research at the 2021 MRA Scientific Retreat.
Despite the tremendous progress made in the last decade in treating melanoma, approximately one third of patients don’t benefit from currently approved therapies. This includes the majority of people with rare forms of melanoma — melanomas that develop in the eye, nailbeds, palms, soles of the feet, and various mucosal membranes. Patients facing these subtypes tend not to respond as well to current treatments as patients with cutaneous melanomas that arise on sun-exposed skin. But as investigators continue to chip away at understanding what causes these melanomas and how they differ from UV-driven cutaneous melanomas, the hope is the better understanding they reap will be sown into improved treatments for these patients. Researchers explored both the challenges and the opportunities for rare melanoma research at the 2021 MRA Scientific Retreat.